REQUIREMENTS :
Agarose powder (high melting point, low EEO), Tris Borate EDTA buffer at pH 8.0, Ethidium bromide stock : 10 mg/ml, Loading buffer, Hand gloves, Electrophoresis apparatus, Pasteur pipette, Plasmid DNA sample.
PROCEDURE :
1. Add the correct amount (0.8 to 1%) of powdered agarose to a measured quantity of electrophoresis buffer, just sufficient to cover the acrylic electrophoresis tray.
2. Heat the slurry in a boiling water bath or in a microwave oven till the agarose dissolves.
3. Cool the solution to 50°C, add 10 μl of stock ethidium bromide solution to every 100 ml of the gel. Do not forget to wear gloves while handling ethidium bromide containing media.
4. Using a Pasteur pipette, seal the edges of a clean, dry, UV transparent plate with a small quantity of agarose gel or use in-situ gel preparation system.
5. When the seal is set. Position and clamp the comb near one end of the tray. Check to see that there is 0.5 to 1.0 mm of distance between the bottom of
the cornb teeth and the base of the tray.
6. Position the tray properly in the electrophoresis tank, pour warm agarose solution to-cover the tray properly and allow the ge! to set for 30 to 45 minutes at room temperature.
7. Carefully remove the comb taking care that the sample wells remain properly formed and sealed at the base.
8. Add just enough electrophoresis buffer to the tank to cover the gel to a depth of about 1mm.
9. 5ul of dye solution consisting of bromophenol blue (0.07%), SDS (7%) and sucrose (10%) in water is added to the DNA sample. Gently mix 5 μl of plasmid DNA sample with 2 μl of loading buffer.
10. Using a disposable micropipette or a Pasteur pipette, load the sample into the slots of the submerged gel. As the DNA sample mixture has a higher density than the buffer, sample sinks into the applied slot.
11. Connect the apparatus. Ensure that the sample slots are towards the cathode.
12. For better resolution apply a voltage of 50V for a 3-inch x 3-inch tray (A voltage of 100V may be applied for a fast run).
13. Allow to run for 20 to 30 minutes taking care that the tracking dye bromophenol blue from the loading buffer does not get overrun.
14. Switch off the electric supply, gently decant the buffer from the surface of the tray and expose the gel (on a UV transparent tray) directly to UV light. (If UV opaque acrylic tray is used for electrophoresis, careful removal of gel is necessary before UV exposure).
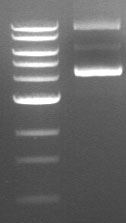
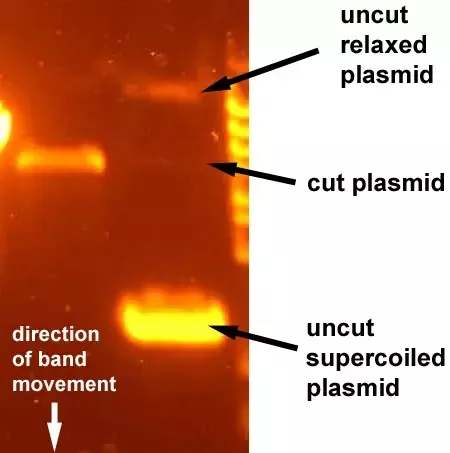
15. The DNA bands fluoresce bright orange under the transilluminator.
Points to note :
1. Always wear gloves while handling ethidium bromide and ethidium bromide containing gels and buffers as ethidium bromide is a very powerful mutagen.
2. Migration of bromophenol blue from loading buffer is 2.2 folds faster than xylene cyanol. Band containing xylene cyanol corresponds to DNA containing 4 kb DNA whereas band moving with bromophenol blue corresponds to DNA containing 300 base pairs.
3. λ-DNA digested with specific restriction endonucleases give fragments of increasing length which may be used for obtaining molecular weight marker ladder in one of the lane.
Appendix :
1. TBE buffer at pH 8.0 :
Prepare a concentrated stock containing the following ingredients per litre :
Tris 108 gm, Boric acid 55 gm, EDTA 7.4 gm, Heat to dissolve. Dilute 1:10 before use. Store at RT.
2. Ethidium bromide :
Add 1 gm of ethidium bromide per 100 ml of distilled water.
Stir on a magnetic stirrer for several hours to ensure that the dye has dissolved.
Wrap the container in aluminium foil or transfer to a dark bottle.
Store at 4°C.
3. Loading buffer (type 1) :
0.25% bromophenol blue, 0.25% xylene cyanol, 40% sucrose in water. Store at 4°C.
4. Loading buffer (type 2) :
0.25% bromophenol blue, 0.25% xylene cyanol, 30% glycerol in water. Store at 4°C.
Result:
Precautions :
1. Wear proper fitting heat-resistant gloves while preparing and pouring agarose gel.
2. If UV-transilluminator is used ensure that a UV proof full face shield is on.
3. Ensure that all the glassware is clean before preparing agarose.
4. Prepare agarose gel in a stirrer hot plate or microwave, do not over boil.
5. Take extra precaution while handing EtBr, which is highly carcinogenic.
4. 0.2 to 0.5 μl of sample DNA is usually loaded per 0.5 cm slot.
