4. Estimation of glucose :
The oldest method of glucose estimation is by its reducing action on cupric ions in alkaline solution (with heat) due to the presence of the aldehyde group. This leads to the formation of cuprous oxide (a red-coloured precipitate). The method is still applied in the screening of urinary glucose. Based on the reducing action of glucose two methods were developed - Folin-Wu and the Somogyi-Nelson method - which are still considered the standard methods in developing countries.
It was later established that these methods are non-specific for glucose and there are other substances (such as glutathione / ergothioneine, galactose, fructose, creatinine, urea, uric acid and others) that have the same reducing property. Hence the methods were referred to as 'glucose as reducing substance' as different from 'true glucose' which is specific towards glucose. The former value is found to be 10-20 higher than the true glucose value.
Another method of non-specific glucose determination is the o-toluidine method which is more convenient, reproducible and closer to the 'true glucose' value than the Folin-Wu method. The o-toluidine method does not require proteinfree filtrate (from serum) which makes it easy to perform. True glucose is determined only by the enzymatic method. Three methods are in common use - the glucose dehydrogenase, glucose oxidase and the hexokinase method. The normal ranges for the o-toluidine method are essentially the same as those for the enzymatic method, since these methods give similar results. In patients with uraemia, however, higher values are obtained with the o-toluidine method.
Of all the methods, only the enzymatic methods are specific while others are non-specific. The o-toluidine method gives results close to the enzymatic methods and does not represent 'reducing substances' because the procedure is based on a condensation reaction instead of the reducing action involving the same organic group present in glucose - aldehyde.
Glucose oxidase (β-D-glucose:oxygen 1-oxidoreductase, EC 1.1.3.4) catalyses the oxidation of β-D-glucose to D-glucono-1,5-lactone and hydrogen peroxide, using molecular oxygen as the electron acceptor. Glucose oxidase is found in the growth medium of Penicillium notatum. The lactone is then slowly hydrolysed to D-gluconic acid.
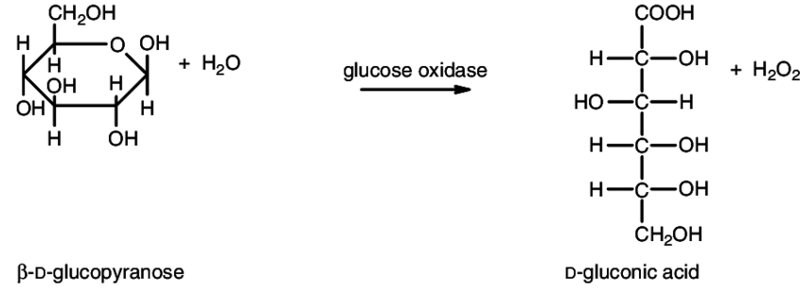
The enzyme is specific for β-D-glucanopyranose, but most enzyme preparations contain mutarotase, which catalyses the interconversion of the α and the β forms.
D-mannose and D-xylose are hydrolysed by the enzymes at about one-hundredth the rate of rate of D-g!ucose and the other common sugar affected is D-galactose which is hydrolysed at about one thousandth the rate. The method is therefore highly specific for glucose.
Glucose is oxidized by the enzyme glucose oxidase (GOD) to gluconic acid with the liberation of hydrogen peroxide, which is converted to water and oxygen by the enzyme peroxidase (POD). Phenol, an oxygen acceptor, takes up the oxygen and combines with the chromogenic substance, 4-aminoantipyrine to form a pinkish-red quinone chromogen which can be measured at 500-515nm.
5. Interfering substances :
Glucose oxidase is inhibited by high concentrations of uric and ascorbic acids, bilirubin, glutathione, creatinine, l-cysteine, L-dopa, dopamine, methyldopa, and citric acid. Although the method is very specific for glucose, high concentrations of reducing substances (e.g. ascorbate) interfere by competing with the chromogen for the liberated oxygen, thus causing false low values of glucose. Hence, patients with vitamin C therapy (ascorbic acid) may yield low values of glucose.
Haemoglobin can also interfere by causing a premature decomposition of hydrogen peroxide, thus giving false low results. If the specimen if haemolysed, icteric (jaundiced with yellow-green colour) or lipaemic, use protein-free filtrate with the test.
6. Specimen :
Measurements of glucose are critical to the diagnosis and management of diseases affecting carbohydrate metabolism. Glucose is measured in whole blood, plasma, serum, cerebrospinal fluid, pleural fluid, and urine for a variety of diagnostic and management purposes.
The standard clinical laboratory analysis of glucose is performed on plasma or serum derived from a phlebotomy specimen. Glycolysis causes plasma glucose to decline over time. A specimen is appropriate for glucose analysis if serum or plasma'is separated from the cells within 30 minutes. If plasma is in contact with cells for much longer than 30 minutes, a preservative such as sodium fluoride that inhibits glycolysis should be added. However, even with the use of fluoride, plasma glucose will decline in the first hour after blood collection.
A serum specimen is recommended for this procedure. For plasma and whole blood, venous blood is collected in tubes or bulbs containing dried sodium fluoride and oxalate and mixed by repeated inversions. The tube is centrifuged to separate the plasma which is then transferred into a clean, dry test tube for the test. Protein-free filtrate (PPF) is required for whole blood and for specimens which are haemolysed, icteric or lipaemic.
1. Take 0.9 ml of 4% TCA (w/v) in a large-sized test tube (19 mm x 150 mm) which helps in mixing.
2. Add 0.1 ml of the test specimen.
3. Mix the contents of the tube.
4. Wait for the next 5 minutes to precipitate the protein.
5. Centrifuge for 5 minutes (2500 rpm) or until the supernatant is clear.
6. Use 0.1 ml of the clear supernatant for testing.
7. Additional information :
a. The glucose oxidase method is not directly applicable to urine specimens, owing to high concentrations of enzyme inhibitors present.
b. Rubber tubings, such as those in pipettors have been found to interfere with colour development and hence should be avoided.
c. Because of high dilutions prior to analysis, common drugs do not have any appreciable effects.
d. Anticoagulants, although they do not affect the results seriously, should be avoided. NaF + oxalate may be used as preservative; thymol inhibits the reactions and should not be used.
