B. Translation - protein synthesis :
Translation is the mechanism in which codons of mRNA are translated and specific amino acids in a sequence form a polypeptide on ribosomes.
All types of proteins are synthesised by the cell, within itself (i.e. intracellularly).
Process of translation requires amino acids, mRNA, tRNA, ribosomes, ATP, Mg++ ions, enzymes, elongation, translocation and release factors.
i. Amino acids form raw material for protein synthesis.
About 20 different types of amino acids are known to form proteins. These are available in the cytoplasm.
ii. DNA controls synthesis of proteins having amino acids in specific sequence.
This control is possible through transcription of m-RNA. Genetic code is specific for particular amino acid.
iii. RNAs serve as intermediate molecules between DNA and protein.
iv. Ribosomes serve as site for protein synthesis. Each ribosome consists of large and small subunits. These subunits occur separately in cytoplasm. Only during protein synthesis, these two subunits get associated together due to Mg++ ions. A ribosome has one binding site for m-RNA and 3 binding sites for t-RNA. They are P site (peptidy t-RNA site), A site (aminoacyl – t-RNA site) and E site (exit site). Only first t- RNA- amino acid complex, directly enters P site of ribosome.
In Eukaryotes, a groove is present between two subunits of ribosomes. It protects the Polypeptide chain from the action of cellular enzymes and also protects mRNA from the action of nucleases.
Mechanism of translation (i.e. synthesis of polypeptide chain) :
It involves three steps :
i. Initiation,
ii. Elongation,
iii. Termination
1. Initiation of Polypeptide chain :
Activation of amino acids
Activation of amino acids is essential before translation initiates for which ATP is essential.
In presence of an enzyme amino acyl tRNA synthetase the amino acid (AA) molecule is activated and then each amino acid is attached to the specific tRNA molecule at 3' CCA end to form aminoacyl-tRNA complex.
The reaction needs ATP. This process is called charging of tRNA or aminoacylation of tRNA.
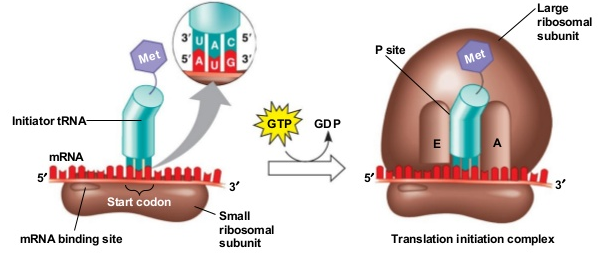
Formation of Initiation complex
a. Small subunit of ribosome binds (attaches) to the m-RNA at 5' end. Initiator codon, AUG is present on m-RNA which initiates the process of protein synthesis (translation). Initiator t- RNA binds with initiation codon (AUG) by its anticodon (UAC) through hydrogen bonds. It carries activated amino acid methionine (in Eukaryotes) or formyl methionine (in prokaryotes).
b. Now the large subunit of ribosome joins with the smaller subunit, that requires Mg++ ions.
c. Initiator charged t-RNA (with activated amino acid methionine) occupies the P-site of ribosome and A-site is vacant.
2. Elongations of polypeptide chain :
During this process, activated amino acids are added one by one to first amino acid (methionine). Amino acid is activated by utilising energy form ATP molecule. This amino acid binds with amino acid binding site of t-RNA- This results in formation of t-RNA- amino acid complex. Addition of Amino acid occurs in 3 Step cycle -
a. Codon recognition- Amino acyl t- RNA molecule enters the ribosome at A-site. Anticodon binds with the codon by hydrogen bonds.
b. Amino acid on the first initiator t-RNA at P-site and amino acid on t-RNA at A-site join by peptide bond. Here enzyme Ribozyme acts as a catalyst. At this time first tRNA at 'P' site is kicked off.
c. Translocation- The t- RNA at A-site carrying a dipeptide at A-site moves to the P-site. This process is called translocation. In translocation, both the subunits of ribosome move along in relation to tRNA and mRNA. Hence, tRNA carrying dipeptide now gets positioned at 'P' site of ribosome, making 'A' site vacant. At this site, then next charged tRNA molecule carrying amino acid will be received. During this process, first uncharged tRNA is discharged from E-site.
This process is repeated as amino acids are added to Polypeptide. It takes less than 0.1 second for formation of peptide bond. Third charged t-RNA with its amino acid, arrives at A-site of ribosome. Anticodon and codon bind by hydrogen bond. Polypeptide bond is formed. Second t-RNA is discharged from P-site to E-site and leaves the ribosome.
So the events like arrival of t-RNA- amino acid complex, formation of peptide bond, ribosomal translocation and removal of previous tRNA, are repeated. As ribosome move over the mRNA, all the codons on mRNA are exposed one by one for translation.
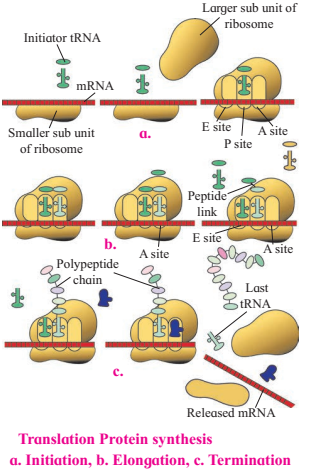
3. Termination and release of polypeptide:
At the end of m-RNA, there is a stop codon (UAA/ UAG/ UGA). It is exposed at the A-site. It is not read and joined by anticodon of any t-RNA.
The release factor binds to the stop codon, thereby terminating the translation process.
The Polypeptide is now released in the cytoplasm. Two subunits of Ribosome dissociate and last tRNA is set free in the cytoplasm.
m-RNA also has some additional sequences that are not translated and are referred as untranslated regions (UTR).
The UTRs are present at both 5'-end (before start codon) and at 3'-end (after stop codon). They are required for efficient translation process.
Finally mRNA is also released in the cytoplasm. It gets denatured by nucleases immediately. Hence mRNA is short -lived.
